Prostate 8
Background and Challenge
Many prostate cancer survivors experience persistent health-related quality of life issues. These challenges can significantly impact daily living and overall well-being. The UCSF Prostate 8 site was part of a randomized clinical trial aimed at testing whether providing tailored information to prostate cancer survivors can influence behaviors and improve outcomes, with a mission to optimize patients’ quality of life during treatment and beyond.
SOM Tech collaborated with Dr. Kenfield and Dr. Van Blarigan in the Department of Urology to support the project.
Driving Behavior Change Through Resources and Support
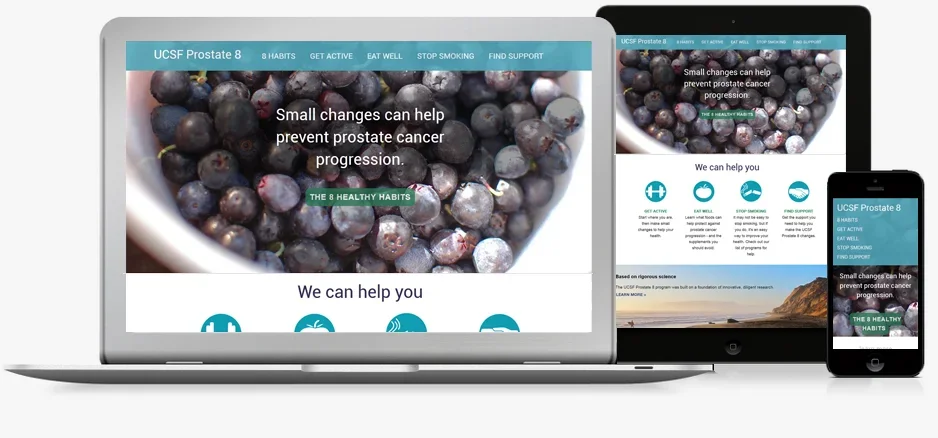
We set out to establish a unique brand identity that would create a visually strong platform while providing a flexible foundation for future Urology initiatives. Patient advocate feedback played a central role, informing website updates and new features to ensure the content reflected real needs and priorities. We emphasized patient-centered language and intuitive navigation, making the site engaging, accessible, and easy to follow.
Brand identity
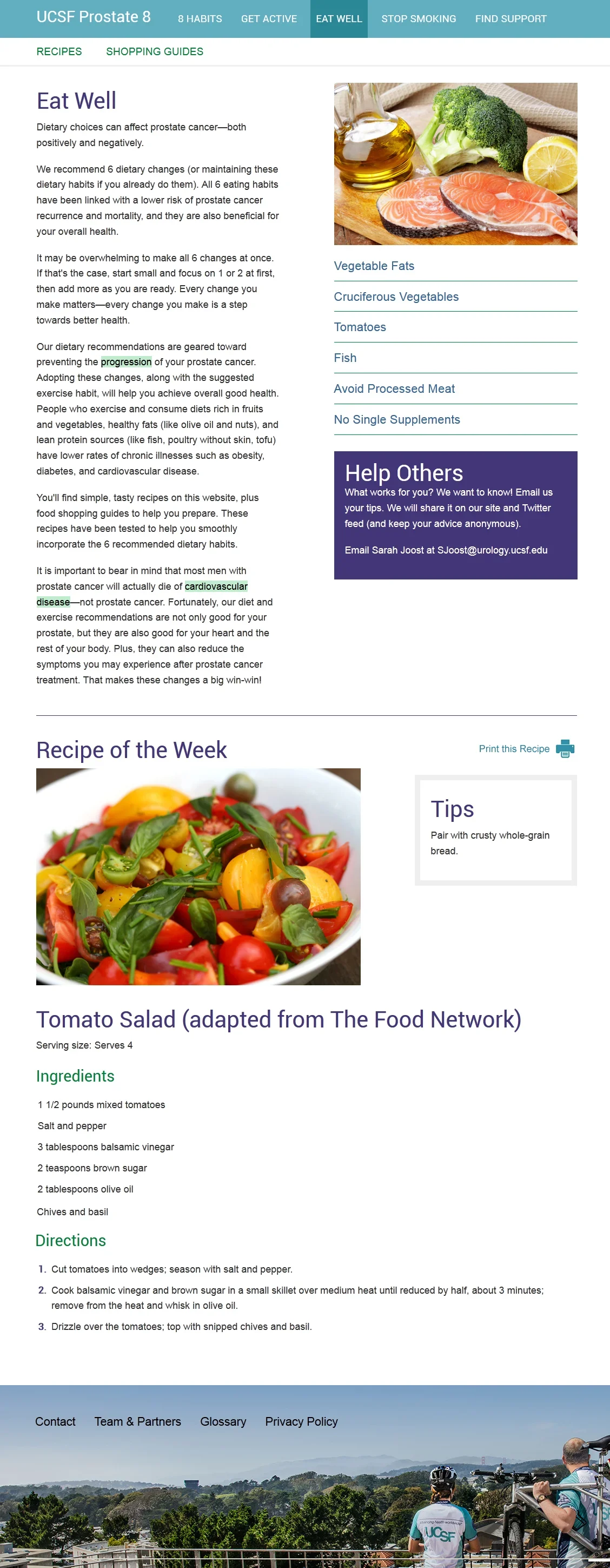
To further support patients, we designed clear goal-setting tools grounded in research-backed recommendations and integrated Twilio messaging to deliver timely reminders and motivational support. Handouts and checklists were also developed for easy reference during everyday activities like shopping. Throughout the design, we encouraged users to seek guidance and support, ensuring the platform not only informed but actively empowered patients in their health journeys.
Home page
Eat Well: recipe and shopping guides
My Work
The pilot randomized controlled trial (Prostate 8) showed the intervention was feasible and acceptable and had positive behavioral outcomes. After the study, the site was made publicly available to participants.
The Prostate 8-II trial was a ten-year, four-arm randomized study of about 200 men who had radical prostatectomy. It tested diet, exercise, or combined interventions delivered through a web portal, text messages, coaching, and practical tools. Participants were followed for 24 months to track biochemical recurrence, biomarkers, quality of life, and other patient-reported outcomes.
Literature:
Feasibility, Acceptability, and Behavioral Outcomes from a Technology-enhanced Behavioral Change Intervention (Prostate 8): A Pilot Randomized Controlled Trial in Men with Prostate Cancer “Results and limitations: At baseline, men in both arms met a median of three targeted behaviors. Sixty-four men (n=32 per arm) completed the study; 88% completed 12-wk assessments (intervention, 94%; control, 82%). Intervention participants wore their Fitbits a median of 82d (interquartile range [IQR]: 72-83), replied to a median of 71% of text messages (IQR: 57-89%), and visited the website a median of 3d (IQR: 2-5) over 12wk. Median (IQR) absolute changes in the P8 score from baseline to 12wk were 2 (1, 3) for the intervention and 0 (-1, 1) for the control arm. The estimated mean score of the intervention arm was 1.5 (95% confidence interval: 0.7, 2.3) higher than that of the control arm at 12wk (ANCOVA p<0.001). Changes were driven by diet rather than exercise. Limitations include self-reported diet and exercise data.”
Collaborated with stakeholders to understand target users content and style preferences iterated based on patient feedback
Wireframed and designed interactions
Built prototypes
Designed website, icons, and visual identity
Created marketing collateral—handouts and postcards
Developed style guide and collaborated with vendors